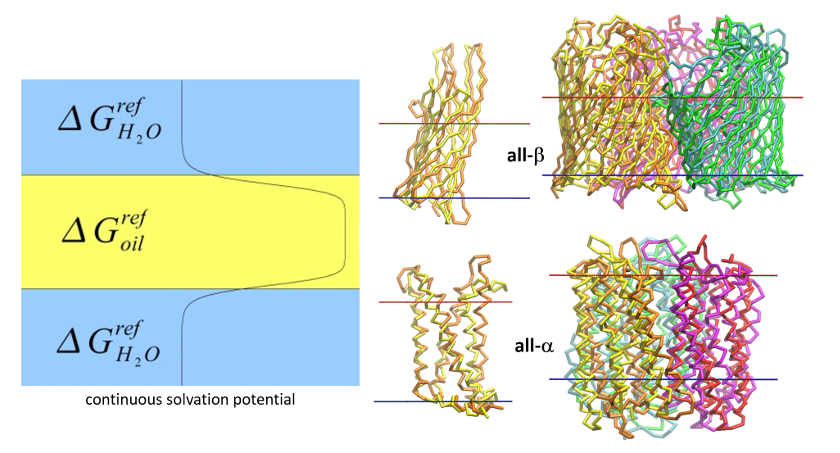
Structures of proteins and their complexes modeled by BIOmodeling group available for downloading.
| Publications | Models | Figures |
|---|---|---|
| S. Yuan, U. Ghoshdastider, B. Trzaskowski, D. Latek, A. Debinski, W. Pulawski, R. Wu, V. Gerke, S. Filipek, The role of water in activation mechanism of human N formyl Peptide Receptor 1 (FPR1) based on molecular dynamics simulations, PLOS ONE (2012) 7, e47114. doi: 10.1371/journal.pone.0047114. | Formyl Peptide Receptor 1 (FPR1) with agonist fMLF with antagonist tBocMLF. The models contain water molecules within a distance < 3 Å from the complex. |  Agonist fMLF located deep in binding site of FPR1.
Agonist fMLF located deep in binding site of FPR1. |
| T.M. Stepniewski, S. Filipek, Non-peptide ligand binding to the formyl peptide receptor FPR2 - a comparison to peptide ligand binding modes, Bioorg. Med. Chem. (2015) 23, 4072-4081. doi: 10.1016/j.bmc.2015.03.062. | Model of free FPR2 and a complex of FPR2 with agonist fMLFK. |  Zones in the orthosteric binding site of FPR2.
Zones in the orthosteric binding site of FPR2. |
THE LATEST NEWS
- July 2023.GS-SMD web server
for SMD simulations of γ-secretase complex.
Publication in Nucleic Acids Research 2023 Web server issue.
- August 2022.COGRIMEN
- June 2021.GPCRsignalOur new service GPCRsignal was recently published in NAR 2021, W1.